Luminol
-
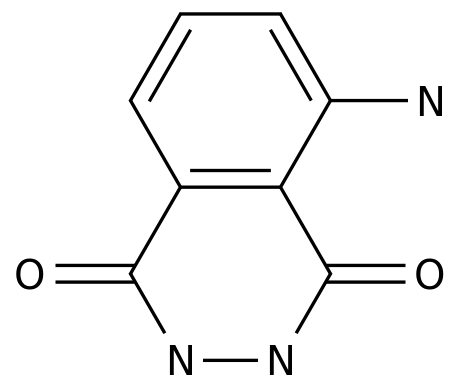
Luminol
structure -
-
CAS No:
521-31-3
-
Formula:
C8H7N3O2
-
Chemical Name:
Luminol
-
Synonyms:
1,4-Phthalazinedione,5-amino-2,3-dihydro-;5-Amino-2,3-dihydro-1,4-phthalazinedione;3-Aminophthalhydrazide;3-Aminophthalic acid hydrazide;3-Aminophthalic hydrazide;Luminol;5-Amino-1,4-dihydroxyphthalazine;NSC 5064;Diogenes reagent
- Categories:
-
CAS No:
Description
Luminol(Diogenes reagent) is a versatile chemical that exhibits chemiluminescence, with a striking blue glow, when mixed with an appropriate oxidizing agent.
O-aminophthalyl hydrazide appears as yellow crystals or light beige powder. (NTP, 1992)
O-aminophthalyl hydrazide appears as yellow crystals or light beige powder. (NTP, 1992)|5-Amino-2,3-dihydro-1,4-phthalazinedione. Substance that emits light on oxidation. It is used in chemical determinations.
Characteristics
91.74000
0.3
O-aminophthalyl hydrazide appears as yellow crystals or light beige powder. (NTP, 1992)
1.61 g/cm3
330.5 °C
621.9ºC at 760 mmHg
329.9ºC
1.649
H2O: <0.1 g/100 mL at 19 ºC;less than 1 mg/mL at 66° F (NTP, 1992)
0-6ºC
Insoluble in water.
Amides and Imides
Oxidation of O-AMINOPHTHALYL HYDRAZIDE is accompanied by a striking emission of light. (NTP, 1992). It is incompatible with strong oxidizing agents, strong reducing agents, strong acids, and strong bases [Sigma-Aldrich MSDS].
Safety Information
NONH for all modes of transport
3
R22; R36/37/38
S26-S37/39
TH8890060
Xn
Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, strong bases, strong reducing agents. Emits light on reaction with oxidizers.
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501
H315
Flash point data for this material are not available, but it is probably combustible. (NTP, 1992)
|Warning|H302 (12.96%): Harmful if swallowed [Warning Acute toxicity, oral]|P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501|Aggregated GHS information provided by 55 companies from 10 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.|H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]|P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501|Aggregated GHS information provided by 45 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Fires involving this material can be controlled with a foam, carbon dioxide or Halon extinguisher. (NTP, 1992)
SMALL SPILLS AND LEAKAGE: If you spill this chemical, FIRST REMOVE ALL SOURCES OF IGNITION, then dampen the solid spill material with toluene, then transfer the dampened material to a suitable container. Use absorbent paper dampened with toluene to pick up any remaining material. Your contaminated clothing and absorbent paper should be sealed in a vapor-tight plastic bag for eventual disposal. Solvent-wash all contaminated surfaces with toluene followed by washing with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned. STORAGE PRECAUTIONS: You should store this material in a refrigerator. (NTP, 1992)
RECOMMENDED RESPIRATOR: Where the neat test chemical is weighed and diluted, wear a NIOSH-approved half face respirator equipped with an organic vapor/acid gas cartridge (specific for organic vapors, HCl, acid gas and SO2) with a dust/mist filter. (NTP, 1992)
Drug Information
Compound such as LUMINESCENT PROTEINS that cause or emit light (PHYSICAL LUMINESCENCE). (See all compounds classified as Luminescent Agents.)|Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
ACUTE/CHRONIC HAZARDS: When heated to decomposition this compound emits toxic fumes. (NTP, 1992)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop. SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment. INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing. INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
Luminol
Luminol Use and Manufacturing
To a three-necked flask was added 5 gl0 wtpercent palladium content meter) palladium on carbon, Stir evenly after heating to 35 ° C, A solution of 260 g of 85 wtpercent aqueous solution of formic acid was slowly added dropwise with stirring, The dropping time was 2 hours, and after the dropwise addition, Continue to react for 3 hours, the point plate to confirm 3-nitro phthalic acid hydrazide has been completely consumed, The pH was adjusted to 3-4 with 35 wtpercent concentrated hydrochloric acid and heated to 100 ° C, Hot filter, the filtrate cooled to l ° C, a large number of precipitation, Precipitate out completely after filtration, the filtrate for organic solvent recovery, The filter cake was washed with 300 ml of deionized water and dried to give 182 g of luminol (3-aminophthalohydrazide).
Detection of copper, iron, peroxides, cyanides. Location of blood on forensic samples.
1,4-Phthalazinedione, 5-amino-2,3-dihydro-: ACTIVE
Computed Properties
Molecular Weight:177.16
XLogP3:0.3
Hydrogen Bond Donor Count:3
Hydrogen Bond Acceptor Count:3
Exact Mass:177.053826475
Monoisotopic Mass:177.053826475
Topological Polar Surface Area:84.2
Heavy Atom Count:13
Complexity:254
Covalently-Bonded Unit Count:1
Compound Is Canonicalized:Yes
Recommended Suppliers of Luminol
-
![China]() CN
CN
4 YRS
Business licensedTrader Supplier of 3,5-Dinitrobenzoic acid,3,5-Din itrobenzoyl chloride,Sodium diatrizoate hydrate,Zinc-5-nitroisophthalate,3-Nitrophthalonitrile,4-Nitrophthalonitrile,Luminol (3-Aminophthalic hydrazide),3-Methyl-4-nitrobenzoic acid,3-Nitrophthalic acid,3-Nitrophthalimide,3-Nitrophthalic anhydride,Dimethyl 3-nitrophthalate,2-Methyl-6-nitro aniline,3-Nitrophthalhydrazide,4-Nitrophthalimide,4-Nitrophthalic acid,4-Nitrophthalic anhydride,4-Nitrosophenol,p-Benzoquinone dioxime,4-Methyl-3-nitrobenzoic acid,4-Chloro-3,5-dinitrobenzoic acid,Isobutyl 4-chloro-3,5-dinitrobenzoate,5-Nitroisophthalic acid,5-Aminoisophthalic acid,3,5-Diaminobenzoic acid,2-Chloro-5-nitrobenzoic acid,4-Chloro-3-nitrobenzoic acid,4-Nitro-N-methylphthalimide,Dimethyl 5-nitroisophthalate,Dimethyl 5-aminoisophthalate,3-Aminophthalimide,3-Methyl-2-nitrobenzoic acid,2-Amino-3-methylbenzoic acid,2-(4-Chloro-3-nitrobenzoyl) benzoic acid,2-(3-Amino-4-chlorobenzoyl)benzoic acid,3-Aminophthalic acid,4-Aminophthalic acid,3-Amino-4-methylbenzoic acid,4-Methyl-3-nitrobenzoate,Methyl 3-amino-4-methylbenzoate,Methyl 3-methyl-2-nitrobenzoate,3,5-Dinitrobenzonitrile,4-Aminophthalimide,4-Amino-N-methylphthalimide,3,5-DinitrobenzamideInquiryUnit Price: $121.4-139.2 /KG FOBCAS No.: 521-31-3Grade: Pharmacy GradeContent: 98.00% -
![China]() CN
CN
3 YRS
Business licensed Certified factoryManufactory Supplier of Minoxidil,Paracetamol,Diclofenac sodium,fenbendazole,IvermectinInquiryCAS No.: 521-31-3Grade: Daily ChemicalContent: 0.00% -
![United States]() US
US
5 YRS
Business licensed Certified factoryManufactory Supplier of Coenzymes,Inhibito,Enzymes,Zymogens,Substrates -
![China]() CN
CN
9 YRS
Business licensed Certified factoryManufactory Supplier of Biological Buffer Chincal Reagent Medical interm -
![China]() CN
CN
4 YRS
Business licensedDistributor Supplier of Nutrition supplement material,Pharmaceutical raw material,Chemical raw materialInquiryCAS No.: 521-31-3Grade: Industrial GradeContent: 98%
Learn More Other Chemicals
-
2-Bromophenacyl bromide, 90%
49851-55-0
-
1H-Indazol-7-ol
81382-46-9
-
6-Chloro-4-forMyl-nicotinic acid
1031433-06-3
-
3-Hydroxy-2,4,5-trifluorobenzoic acid Formula
116751-24-7
-
2-Methyl-1-heptene Formula
15870-10-7
-
1-(3,5-Dinitrophenyl)ethanone Formula
14401-75-3
-
α-Amino-3-bromobenzeneacetic acid Structure
79422-73-4
-
6-(BROMOMETHYL)-1,3-BENZOTHIAZOLE,97% Structure
499770-85-3
-
What is ethyl 5-hydroxy-2-Methylnicotinate
60390-47-8
-
What is 2-bromo-1-(4-bromo-3-fluorophenyl)ethanone
1003879-02-4